

Document Type : Research Paper
1. Introduction
Vapor-liquid equilibrium modeling of natural gas at high pressure is essential in the design of many processes such as dehydration and natural gas treatment. To design a chemical process, the equations of state have been widely used for vapor-liquid equilibrium calculations. Using local composition models, the various mixing rules have been developed such as Huron and Vidal [1], Modified Huran and Vidal second order [2], and Wong and Sandler [3] etc. These mixing rules have been widely applied to modeling a variety of systems using energy interaction parameters that should be adjusted by the optimization of vapor-liquid equilibrium data. The predictability of these mixing rules is a matter of controversy for multicomponent mixtures.
The cubic equation of state such as van der Waals (vdW), Soave-Redlich-Kwong (SRK) and Peng-Robinson (PR) have been widely used for the prediction of phase behavior of nonpolar and slightly polar systems. Using the cubic equation of state, one-fluid theory with quadratic mixing rules have been applied for the calculation of vapor-liquid and liquid-liquid equilibrium of multicomponent mixtures.
Since Huron and Vidal [1] who proposed a new mixing rule known as EOS/AE model, the numerous mixing rules [2-7] using the activity coefficient models such as Wilson [8] and Universal-Quasi-Chemical (UNIQUAC [9]) have been developed. Among these successful attempts, several mixing rules have been developed such as Orbey-Sandler [10], Twu-Sim-Tassone (TST [11]), van der Waals - local composition (VWLC I and VWLC II [12]) among which van der Waals one-fluid mixing rule was recovered. In the VWLC mixing rules, the local composition was directly incorporated into the cubic equation of state (CEOS), in a similar manner as done by Heyen [13]. In this study, two new mixing rules of LCEOS1 and LCEOS2 were developed so that the energy interaction parameters were expressed by attractive and repulsive parameters of the CEOS. The LCEOS1 mixing rule was developed by the combination of the cubic equation of state with excess molar internal energy using local composition concept without incorporating a pressure reference; however, this mixing rule doesnot reduce to classical quadratic mixing rule. On the other hand, the LCEOS2 can be reduced to the VdW mixing rules. The results of these two new mixing rules were compared with van der Waals one-fluid theory as a basis.
2. The VdW Mixing Rule
One of the generalization forms of the two parameters vdW type of CEOS can be written as:
![]() (1)
(1)
where ![]() and
and ![]() are the numeric constants which are given by the type of EOS. The
are the numeric constants which are given by the type of EOS. The ![]() and
and ![]() are the attractive and repulsive parameters that
are the attractive and repulsive parameters that ![]() so that both aC and b are calculated by critical properties. A variety of alpha function,
so that both aC and b are calculated by critical properties. A variety of alpha function,![]() , has been proposed for the different cubic EOSs. For the mixture of nonpolar compounds, the mixing rules of
, has been proposed for the different cubic EOSs. For the mixture of nonpolar compounds, the mixing rules of ![]() and
and ![]() for VdW one-fluid are as:
for VdW one-fluid are as:
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
This quadratic mixing rule is widely used to predict the nonideal behavior of mixtures at moderate pressures. However, for strongly nonideal systems containing polar and associated components, more precise mixing rules are needed so that the two LCEOS1 and LCEOS2 mixing rules are developed as follows.
3. The LCEOS1 Mixing Rule (Local Composition- Equation of State 1)
Following the classical thermodynamics,
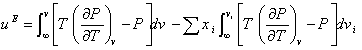 (5)
(5)
and using Eq. (1) and following van Laar’s assumption that the parameters "a" and “b” are independent of temperature [14], by the integration of Eq. (5), the molar excess internal energy was obtained as:
![]() (6)
(6)
where C is the characteristic constant of the cubic equation of state, CEOS, and is expressed as,
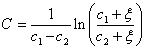 (7)
(7)
![]() (8)
(8)
where the parameter ![]() is always greater than unity and approaches unity at infinite pressure [5]. By substituting the vdW mixing rules of
is always greater than unity and approaches unity at infinite pressure [5]. By substituting the vdW mixing rules of ![]() and
and ![]() into Eq. (6), the excess internal energy for a binary system can be written as:
into Eq. (6), the excess internal energy for a binary system can be written as:
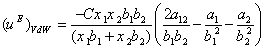 (9)
(9)
Eq. (9) is similar to excess molar Gibbs energy which was developed by van Laar [14]. On the other hand, the ![]() can be expressed by using local composition concept [8] and two-liquid theory [15] as,
can be expressed by using local composition concept [8] and two-liquid theory [15] as,
![]() (10)
(10)
where ![]() is the internal interaction energy between species i and j and
is the internal interaction energy between species i and j and ![]() is the local composition mole fraction of species " i " surrounding central species " j ".
is the local composition mole fraction of species " i " surrounding central species " j ".
A new expression for the local composition is defined as:
![]() (11)
(11)
where,
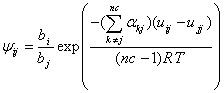 (12)
(12)
where “nc” is number of components in a mixture and ![]() is the nonrandomness parameter of species k surrounding a central species j. As one may consider that for a binary system, Eq. (12) reduces to the simple local composition expression of the NRTL model; however for multicomponent systems the nonrandomness is not the same for each pair. In fact, Eq. (12) assumes that the average nonrandomness of each pair has a unique value for each cell. In another word, one may assume that the coordination number for an individual cell has different values.
is the nonrandomness parameter of species k surrounding a central species j. As one may consider that for a binary system, Eq. (12) reduces to the simple local composition expression of the NRTL model; however for multicomponent systems the nonrandomness is not the same for each pair. In fact, Eq. (12) assumes that the average nonrandomness of each pair has a unique value for each cell. In another word, one may assume that the coordination number for an individual cell has different values.
By combining Eqs. (10) and (11), the molar excess internal energy for a binary system is obtained as:
![]() (13)
(13)
where ![]() . The system is at random state as
. The system is at random state as ![]() approaches zero. By substituting
approaches zero. By substituting ![]() in Eq. (12) and rearranging it, the following relation is obtained:
in Eq. (12) and rearranging it, the following relation is obtained:
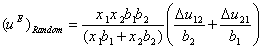 (14)
(14)
On the other hand, the VdW mixing rule resulted from a random mixing [12], thus by equivalency of Eqs. (9) and (14), the following relation for the interaction energy can be obtained:
![]() (15)
(15)
By substituting Eq. (15) into Eq. (12), the following relation is obtained:
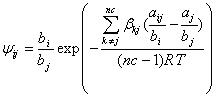 (16)
(16)
where ![]() with a negative value, because both C and
with a negative value, because both C and ![]() have positive values. By the extension of
have positive values. By the extension of ![]() for a multicomponent mixture and using Eq. (16), one can be obtained as follows:
for a multicomponent mixture and using Eq. (16), one can be obtained as follows:
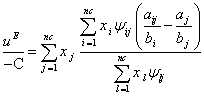 (17)
(17)
Finally, having the function of molar excess internal energy, the molar Helmholtz energy can be obtained by [14]:
![]() (18)
(18)
At very high temperatures, 1/T0®0, we can assume that the components form an athermal mixture. Thus, as a boundary condition, we use the equation of Flory-Huggins combinatorial theory. By assuming the independence of ![]() with respect to temperature [9] and using Eq. (17), the integration of Eq. (18) results the molar excess Helmholtz energy for a binary mixture as:
with respect to temperature [9] and using Eq. (17), the integration of Eq. (18) results the molar excess Helmholtz energy for a binary mixture as:
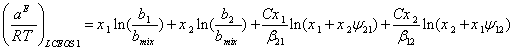 (19)
(19)
The first two terms on the right side are known as the Flory-Huggins model. As one can see, the molar volumes are written in terms of the parameters b1 and b2 from CEOS. The term ![]() can be presented by Eq. (16). Thus, Eq. (19) can be considered as a new Excess Helmholtz energy function that is based on the repulsive and attractive parameters of the CEOS. It should be noted that the assumption of temperature independence of the CEOS parameters, i. e, a and b, in the derivation of Eq. (6) corresponds to the well- known assumption of temperature independence of
can be presented by Eq. (16). Thus, Eq. (19) can be considered as a new Excess Helmholtz energy function that is based on the repulsive and attractive parameters of the CEOS. It should be noted that the assumption of temperature independence of the CEOS parameters, i. e, a and b, in the derivation of Eq. (6) corresponds to the well- known assumption of temperature independence of ![]() in using Eq. (18) to obtain the excess function such as Wilson and UNIQUAC models.
in using Eq. (18) to obtain the excess function such as Wilson and UNIQUAC models.
On the other hand, following the classical thermodynamics, the molar Helmholtz energy can be obtained as [6]:
![]() (20)
(20)
where the value of “C” is presented by Eq. (7). Thus, by combining Eqs. (19) and (20), the LCEOS1 mixing rule is obtained as:
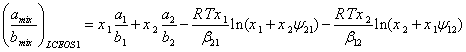 (21)
(21)
Eq. (21) can be generalized for a multicomponent mixture as:
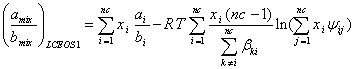 (22)
(22)
As mentioned above, the term ![]() can be presented by Eq. (16). In this study, Eq. (9) was used for
can be presented by Eq. (16). In this study, Eq. (9) was used for ![]() of LCEOS1. The three adjustable parameters of LCEOS1 were
of LCEOS1. The three adjustable parameters of LCEOS1 were ![]() ,
, ![]() and
and ![]() , however one may fix one or two of the parameters and optimize the others depending on the nature of a system. One of the advantages of LCEOS1 is no need to consider a value for
, however one may fix one or two of the parameters and optimize the others depending on the nature of a system. One of the advantages of LCEOS1 is no need to consider a value for ![]() which is hidden in the parameter
which is hidden in the parameter ![]() .
.
4. The LCEOS2 Mixing Rule (Local Composition-Equation of State 2)
The LCEOS1 mixing rule can not be reduced to the VdW mixing rule at random state; however, by combining Eqs. (17) and (6), the LCEOS2 mixing rule is obtained as:
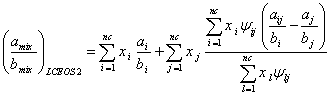 (23)
(23)
Using the relation of second virial coefficient in terms of CEOS parameters as shown by Orbey and Sandler [6], the following relations are adopted for the ![]() of LCEOS2 mixing rule as,
of LCEOS2 mixing rule as,
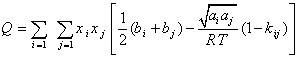 (24)
(24)
![]() (25)
(25)
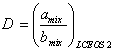 (26)
(26)
In this work, Eqs. (23) to (26) as the LCEOS2 mixing rule were applied for the vapor-liquid equilibrium calculations. The adjustable parameters of both LCEOS1 and LCEOS2 are the same. There is no need to consider a value for ![]() in LCEOS2 mixing rule as such in the LCEOS1 mixing rule. It should be considered that by using Eq. (24), the LCEOS2 mixing rule has the advantage that follows the quadratic dependence of composition for the second virial coefficient. In addition, the other benefit of this mixing rule is to recover VdW one-fluid mixing rule with the assumptions of
in LCEOS2 mixing rule as such in the LCEOS1 mixing rule. It should be considered that by using Eq. (24), the LCEOS2 mixing rule has the advantage that follows the quadratic dependence of composition for the second virial coefficient. In addition, the other benefit of this mixing rule is to recover VdW one-fluid mixing rule with the assumptions of ![]() and
and ![]() .
.
5. Results and Discussion
The processing and treating of natural and synthetic gases are highly important, particularly, at high pressure. These systems consist of various hydrocarbon and nonhydrocarbon components such as alcohols, acid gases and water that one demands to use a precise CEOS for the accurate calculation of vapor-liquid equilibrium at high pressure. To investigate the application of both LCEOS1 and LCEOS2 mixing rules, the bubble pressure of 13 binary systems of hydrocarbon and nonhydrocarbon systems were correlated. In addition, the phase behavior of three binary and two ternary systems including alcohols, hydrocarbon, acid gases and water at high pressure were predicted. These systems are shown in Tables 1 to 3.
In the present study using the EOS/AE approach, the Peng-Robinson EOS [16], with both LCEOS1 and LCEOS2 mixing rules, was used and compared with the results of Wong Sandler-NRTL [3] and VdW one-fluid theory. The adjustable parameters of WS-NRTL mixing rule are ![]() ,
, ![]() and
and ![]() . The single adjustable parameter
. The single adjustable parameter ![]() for VdW EOS was used. The critical properties and acentric factor of pure components were obtained from Poling et al. [17] and Smith and Van Ness [18], except acentric factor of triethylene glycol (TEG). So, using the vapor pressure of TEG [19], the acentric factor of 0.756 was obtained for TEG. For some systems, to cover all of the experimental data at the whole range of temperature, the adjustable parameters were considered as a linear function of temperature as:
for VdW EOS was used. The critical properties and acentric factor of pure components were obtained from Poling et al. [17] and Smith and Van Ness [18], except acentric factor of triethylene glycol (TEG). So, using the vapor pressure of TEG [19], the acentric factor of 0.756 was obtained for TEG. For some systems, to cover all of the experimental data at the whole range of temperature, the adjustable parameters were considered as a linear function of temperature as:
![]() (27)
(27)
where “AP” shows an adjustable parameter, “A” and “B” are the coefficients that were optimized as two adjustable parameters. The advantage of using Eq. (27) allows one to use both mixing rule as a predictive model, so one can perform equilibrium prediction at the other temperatures.
Using Nelder-Mead optimization method, the binary parameters were obtained by fitting the P-x and P-x-y data through the following objective function:
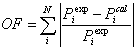 (28)
(28)
where N shows the number of experimental data points. To determine the deviation of the calculated results from the experimental data, the percent average absolute deviation (AAD%) was used as,
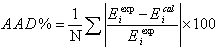 (29)
(29)
where E can be the dew pressure or vapor phase mole fraction.
Table 1: the binary systems used for obtaining the binary parameters.
|
system |
Comp. (1) |
Comp. (2) |
T/K |
Ref. |
|
1 |
CH4 |
H2O |
283.2-303.2 |
[20] |
|
2 |
C2H6 |
H2O |
283.1-313.2 |
[21] |
|
3 |
CH4 |
TEG |
298.15-373.15 |
[22] |
|
4 |
CH4 |
EG |
283.2-303.2 |
[20] |
|
5 |
C2H6 |
EG |
283.2-303.2 |
[20] |
|
6 |
CH4 |
Methanol |
283.2-303.2 |
[20] |
|
7 |
CH4 |
Ethanol |
313.4,333.4 |
[23] |
|
8 |
H2O |
EG |
371.15 |
[24] |
|
9 |
CO2 |
H2O |
313.15-373.15 |
[25] |
|
10 |
CO2 |
TEG |
297.04-322.04 |
[26] |
|
11 |
CO2 |
Methanol |
313.2,313.4 |
[23, 27] |
|
12 |
CO2 |
Ethanol |
313.4,333.4 |
[23] |
|
13 |
H2S |
TEG |
298.15-373.15 |
[22] |
Table 2: The binary systems used for the prediction of phase behavior.
|
System |
Comp. (1) |
Comp. (2) |
T/K |
Ref. |
|
14 |
CH4 |
TEG |
398.15 |
[22] |
|
15 |
H2S |
TEG |
398.15 |
[22] |
|
16 |
C2H6 |
H2O |
323.2 |
[21] |
Table 3: The ternary systems used for the prediction of phase behavior.
|
System |
Comp. (1) |
Comp. (2) |
Comp. (3) |
T/K |
Ref. |
|
17 |
CH4 |
EG |
H2O |
283.2-303.2 |
[20] |
|
18 |
C2H6 |
EG |
H2O |
283.2-303.2 |
[20] |
5.1. The binary systems
Table 4 shows the binary parameters of the 2 mixing rules which were applied for the correlation of the vapor-liquid equilibrium of thirteen binary systems. For the cases which data were available for a single temperature, three adjustable parameters were used, otherwise a linear temperature dependency with two adjustable coefficients for k12 or k21 were used. For binaries 3, 4, 6, 7 and 13 only the three parameters, two coefficients and a single parameter of the LCEOS1 mixing rule were adjusted for the whole range of temperatures. The average absolute deviation in pressure and vapor mole fraction of the binary systems is shown in Table 5.
Table 4: the binary parameters of the LCEOS1, LCEOS2 mixing rules for 13 binary systems.
|
|
LCEOS1 |
LCEOS2 |
||||
|
system |
k12 |
k21 |
β12 |
k12 |
k21 |
β12 |
|
1 |
2.731 |
0.0536 |
-0.111 |
0.6993 |
0.3687 |
-0.0577 |
|
2 |
1.887 |
0.2865 |
-0.09358 |
0.3015 |
0.484 |
-0.0639 |
|
3 |
(0.2403+ 0.004206T) |
k12 |
-0.2938 |
0.373 |
0.8581 |
-0.1285 |
|
4 |
(0.2835+ 0.002978T) |
k12 |
-0.3776 |
(-3.013+ 0.01108T) |
(0.5116 - 0.001068T) |
-0.06631 |
|
5 |
0.8257 |
0.5804 |
-0.135 |
0.5164 |
0.7818 |
-0.1069 |
|
6 |
(-0.02095+0.002344T) |
k12 |
-0.9505 |
1.2 |
0.4116 |
-0.4163 |
|
7 |
( 0.1678+0.001553T) |
k12 |
-0.8993 |
0.547 |
k12 |
-0.3358 |
|
8 |
0.2487 |
0.4029 |
-0.0617 |
-0.1605917 |
0.6272035 |
-0.04864 |
|
9 |
0.9802 |
0.0274 |
-0.0919 |
(-0.4287+ 0.00105T) |
k12 |
0 |
|
10 |
-0.05077 |
0.6673 |
-0.4153 |
0.2345 |
0.2009 |
-0.802 |
|
11 |
-0.04918 |
0.2746 |
-0.1549 |
0.2112 |
0.3947 |
-0.6432 |
|
12 |
-0.07129 |
0.3975 |
-0.6567 |
0.1499 |
0.3749 |
-0.5461 |
|
13 |
(-0.3339+0.001847T) |
k12 |
-0.2816 |
(0.08122- 0.0003906T) |
(-0.2104+ 0.001525T) |
-0.1619 |
Table 5: The percent average absolute deviation in pressure and vapor mole fraction of the first component for the binary systems (the second value at each column shows the deviation for vapor mole fraction).
|
System |
AAD(P) %, AAD(y)% |
||||
|
LCEOS1 |
LCEOS2 |
WS-NRTL |
VdW |
||
|
1 |
CH4+H2O |
5.93 |
11.12 |
10.79 |
25.44 |
|
2 |
C2H6+H2O |
2.19 |
2.55 |
3.63 |
33.95 |
|
3 |
CH4+TEG |
1.28 |
1.17 |
1.83 |
1.64 |
|
4 |
CH4+EG |
4.47 |
4.17 |
3.46 |
4.53 |
|
5 |
C2H6+EG |
7.10 |
6.97 |
8.21 |
6.97 |
|
6 |
CH4+Methanol |
3.22 |
4.88 |
5.57 |
12.58 |
|
7 |
H2O+Ethanol |
0.47, 0.26 |
0.97, 0.14 |
0.55, 0.48 |
5.59, 0.41 |
|
8 |
H2O+EG |
3.64, 9.22 |
4.47, 10.59 |
2.48, 9.41 |
2.59, 8.08 |
|
9 |
CO2+H2O |
3.69, 0.46 |
4.42, 3.16 |
5.01, 3.06 |
4.42, 3.16 |
|
10 |
CO2+TEG |
2.45 |
4.64 |
6.08 |
5.33 |
|
11 |
CO2+Methanol |
3.16, 0.49 |
3.44, 0.61 |
3.23, 0.36 |
4.81, 1.12 |
|
12 |
CO2+Ethanol |
2.4, 0.26 |
2.83, 1.35 |
1.39, 0.156 |
5.34, 0.627 |
|
13 |
H2S+TEG |
5.51 |
6.36 |
5.59 |
12.97 |
Figures 1 to 4 show the results of comparison for the different mixing rules for several binary systems. Figures 1 and 2 show the results for methane and ethane solubility in water at 303.2 K. As one can observe, the calculated data by LCEOS1 mixing rule are in very good agreement with the experimental data. One can see that LCEOS1 mixing rule shows significant difference with respect to the other three mixing rules for (methane + water) system; however, there is no significant difference between the mixing rules of LCEOS1, LCEOS2 for the other systems. It should be noted that the VdW mixing rule presents large deviations from experimental data since it is known that it cannot describe systems of extremely nonideal behavior, such as (hydrocarbon + water) systems. Figure 3 shows the comparison of the three mixing rules for correlating solubility of H2S in TEG at 323.15 K. So, the results of LCEOS1 and WS-NRTL mixing rules were found to be in good agreement with data.
Table 6 shows the results of prediction for the three binary systems. So the results of LCEOS1 mixing rule present better accuracy for methane+TEG and H2S+TEG systems and WS-NRTL shows more accuracy for ethane+water system. Figure 4 shows the predicted results of H2S in TEG at 398.15 K.
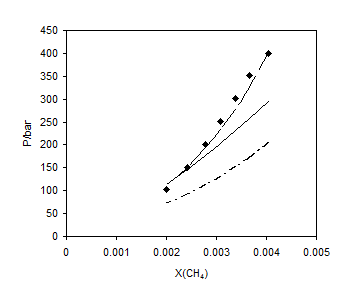
Figure 1. Vapor + liquid equilibrium of methane + water at 303.2 K: ■, experimental data [20]; – – , LCEOS1; —–, LCEOS2; – ۔ , VdW.
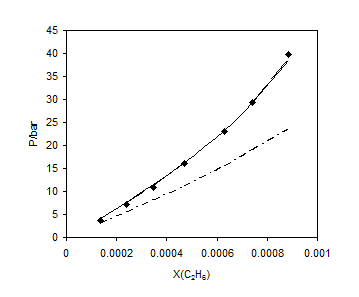
Figure 2. Vapor + liquid equilibrium of ethane + water at 303.2 K: ■, experimental data [21]; —–, LCEOS1; – – LCEOS2; – ۔, VdW.
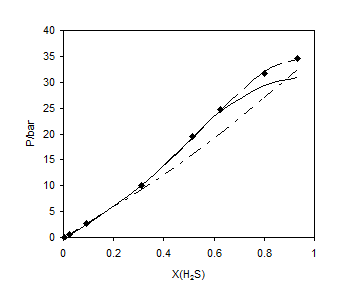
Figure 3. Vapor + liquid equilibrium of H2S + TEG at 323.15 K: ■, experimental data [22]; – –, LCEOS1; —–, LCEOS2; – ۔ , VdW.
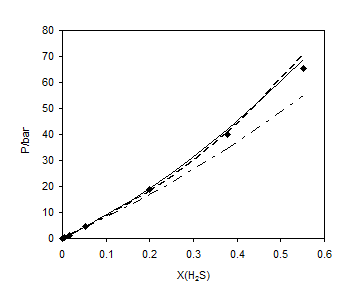
Figure 4. Prediction of Vapor + liquid equilibrium of H2S + TEG at 398.15 K: ■, experimental data [22]; – –, LCEOS1; —–, LCEOS2; – ۔ , VdW.
Table 6: The average absolute deviation in pressure for the prediction of the three binary systems.
|
System |
AAD(P) % |
||||
|
LCEOS1 |
LCEOS2 |
WS-NRTL |
VdW |
||
|
14 |
CH4+TEG |
1.57 |
2.05 |
2.64 |
1.50 |
|
15 |
H2S+TEG |
6.97 |
8.86 |
8.24 |
11.38 |
|
16 |
C2H6+H2O |
5.35 |
4.50 |
3.29 |
64.52 |
5. 2. The ternary systems
A strong mixing rule is the one that not only correlates well with experimental data of binary systems, but also predicts the phase behavior of multicomponent mixtures properly. Tables 7 and 8 show the deviation of the predicted results from the experimental data for the ternary systems of (methane + ethylene glycol + water) and (ethane + ethylene glycol + water), respectively. Using the LCEOS1 mixing rule, the results of prediction for both ternaries are much better than other mixing rules. Figures 5 and 6 show the prediction of methane and ethane solubility in 60% ethylene glycol in the aqueous phase on hydrocarbon free basis at 293.14K, respectively. The results of the LCEOS1 mixing rule were in excellent agreement with the experimental data.
Table 7. Average absolute deviation in pressure for the (vapor + liquid) equilibrium prediction of (methane + ethylene glycol + water) ternary system.
|
AAD(P) % |
||||
|
LCEOS1 |
VdW |
|||
|
T/K |
40% EG |
60% EG |
40% EG |
60% EG |
|
283.2 |
5.86 |
19.39 |
60.35 |
70.36 |
|
293.2 |
8.51 |
5.25 |
64.62 |
70.44 |
|
303.2 |
17.83 |
29.03 |
68.95 |
68.69 |
|
LCEOS2 |
WS-NRTL |
|||
|
T/K |
40% EG |
60% EG |
40% EG |
60% EG |
|
283.2 |
13.55 |
52.40 |
30.89 |
39.79 |
|
293.2 |
9.55 |
48.30 |
29.77 |
34.84 |
|
303.2 |
8.38 |
41.42 |
31.75 |
24.77 |
Table 8: Average absolute deviation in pressure for the (vapor + liquid) equilibrium prediction of (ethane + ethylene glycol + water) ternary system.
|
AAD(P) % |
||||
|
LCEOS1 |
VdW |
|||
|
T/K |
40% EG |
60% EG |
40% EG |
60% EG |
|
283.2 |
22.71 |
15.24 |
56.87 |
71.10 |
|
293.2 |
11.23 |
15.52 |
59.76 |
73.79 |
|
303.2 |
11.46 |
23.96 |
66.50 |
76.06 |
|
LCEOS2 |
WS-NRTL |
|||
|
T/K |
40% EG |
60% EG |
40% EG |
60% EG |
|
283.2 |
25.48 |
59.87 |
23.73 |
30.76 |
|
293.2 |
18.38 |
60.35 |
8.92 |
25.41 |
|
303.2 |
21.39 |
60.80 |
10.07 |
17.35 |
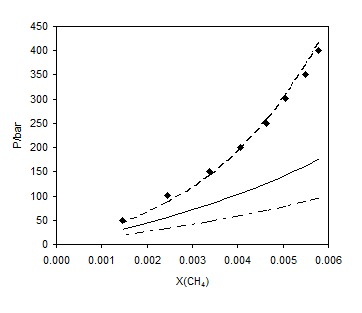
Figure 5. Comparison of different mixing rules for the prediction of methane solubility in solution of 60% ethylene glycol in the aqueous phase on hydrocarbon free basis at 293.2 K; ■, experimental data [20]; – –, LCEOS1; —–, LCEOS2; – ۔ , VdW.
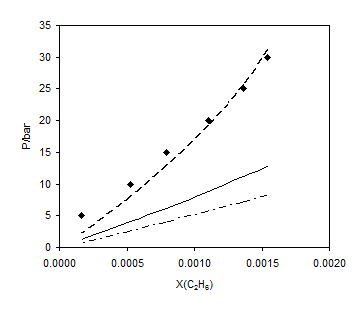
Figure 6. Comparison of different mixing rules for the prediction of ethane solubility in solution of 60% ethylene glycol in the aqueous phase on hydrocarbon free basis at 293.2 K; ■, experimental data [20]; – –, LCEOS1; —–, LCEOS2; – ۔ , VdW.
6. Conclusion
In this study, two new mixing rules, LCEOS1 and LCEOS2, were presented for the correlation and prediction of the vapor-liquid equilibrium behavior of hydrocarbon + nonhydrocarbon systems at high pressure. These mixing rules were based on EOS/ AE approach and energy interaction parameters were calculated by attractive and repulsive parameters of cubic equation of state by the optimization of the experimental data. It should be mentioned that as it is usual in Huron-Vidal and the other mixing rules in this category, the interaction energy parameters were optimized. However, in this study the coupling (interaction) parameters were optimized. Applying the mixing rules LCEOS1, LCEOS2 , WS-NRTL and VdW, the new parameters were obtained for the correlation of thirteen binary systems including CO2, H2S, H2O, CH4, C2H6, EG, TEG. The results were compared and showed that LCEOS1 mixing rule presented better accuracy. Moreover, these mixing rules were used for the prediction of phase equilibrium of three binary and two ternary systems. The comparison of four mixing rules for the ternary systems of CH4 + EG + H2O and C2H6 + EG + H2O demonstrated that the new mixing rule LCEOS1 showed better results so that it is a suitable mixing rule for the modeling of phase behavior of nonideal mixtures. Thus, further testing, accounting for a broader class of systems should be conducted in order to reach a more general conclusion on the power of the new mixing rules presented in this study.
Appendix A: calculation of VLE by the new mixing rules
The phase equilibrium condition for VLE calculation in the φ-approach is expressed as,
![]() (A.1)
(A.1)
The fugacity coefficient of component “ i “ in the mixture can be obtained by the following relation:
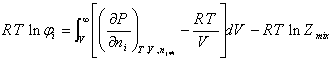 (A.2)
(A.2)
where Zmix is the mixture compressibility factor. By using equation (1) and integration of equation (A.2) one can obtain,
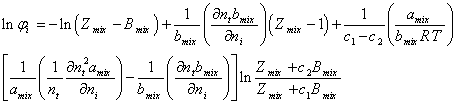 (A.3)
(A.3)
where,
![]() (A.4)
(A.4)
Based on the LCEOS1 mixing rule, the derivatives in equation (A.3) are as follows,
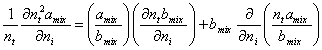 (A.5)
(A.5)
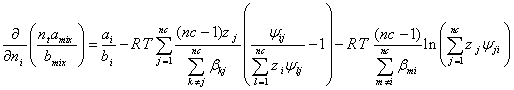 (A.6)
(A.6)
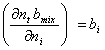 (A.7)
(A.7)
where z is mole fraction of either liquid or vapor phases.
Based on LCEOS2 mixing rule, the derivatives in equation (A.3) are as follows,
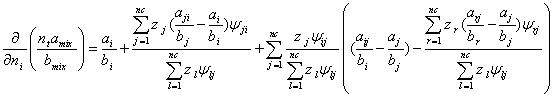 (A.8)
(A.8)
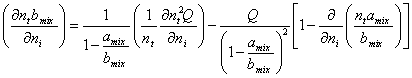 (A.9)
(A.9)
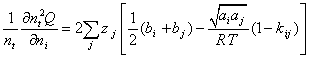 (A.10)
(A.10)