

Document Type : Research Paper
1. Introduction
The current global dependence on petroleum as the major source of energy and lack of short-term substitutes for this form of energy necessitates maximum recovery from active reserves and reactivation of those regarded as depleted oil reservoirs. The challenge to increase the oil recovery from depleted oil reservoirs is a driving force behind the efforts to come up with alternative, cost-efficient and environmentally compatible recovery processes [1]. EOR technologies are of utmost importance as they permit further recovery from depleted or declining oil reserves. A considerable amount of research works go into laboratory investigation, field pilot trials and also large-scale implementation of different EOR methods annually [2-5]. Various EOR methods are being evaluated, including the use of microbial processes in the reservoirs. Basically, microbial enhanced oil recovery (MEOR) is a collection of techniques that utilizes microorganisms and their metabolic products to improve the recovery of crude oil from reservoir rock [6-8]. Besides, MEOR is one of the environmentally compatible technologies that can potentially be implemented with an exceptionally low operating cost. Research and field testing during the last two decades have demonstrated the industrial potential for the exploitation of microorganisms in EOR [9].
There are several scenarios in which MEOR can be implemented in oil fields. The simplest form of MEOR is ex-situ application which uses microbial products rather than living microorganisms themselves to enhance oil recovery [8]. In in-situ methods, microorganisms are used to grow and produce bio-products inside the oil reservoir [9-10]. The benefit of this method is that it eliminates the need of complex upstream facilities for bio-product production and purification. It can also be ensured that a reservoir naturally-occurring microorganism is already adapted to the harsh conditions of temperature and salinity. However, it might be difficult to activate these microbes in sufficient amounts using relatively cheap nutrients.
There are a number of mechanisms through which microorganisms are believed to result in enhanced oil recovery. As one of the most basic mechanisms, profile modification and the subsequently improved sweep efficiency occurs, when the biomass produced by the reproduction of cells blocks certain high permeability flow channels forcing the flow to sweep low permeability zones. Blocking of pores can also be done by biopolymers produced in-situ or introduced by injection [9]. Biosurfactants and bio-emulsifiers produced by microorganisms reduce the IFT between oil and brine, resulting in the displacement of trapped oil blobs or easier movement of it in the pores [11-12].
Wettability is the tendency of one fluid to spread on or adhere to a solid surface in the presence of other immiscible fluids. Wettability of the oil rock formation is important as it controls the location, flow and distribution of fluids within the reservoir rocks [13-14]. The oil recovery is affected by wettability alteration through the effects of metabolite adsorption or the action of adhered microorganisms [11, 15-16]. Biogas production has also been proposed as a mechanism that maintains reservoir pressure and improves the relative permeability [16].
These, along with several other mechanisms can take place individually or simultaneously depending on the type of microorganism. Among the mentioned mechanisms, the production of biosurfactants is specifically attractive as it is able to recover a substantial amount of trapped oil and also result in lower residual oil saturation.
However, many oil reservoirs are anaerobic and have high temperature, salinity and pressure which are often cited as some of the limitations for MEOR process [17]. As a result, this technology requires the microorganisms that are able to withstand difficult reservoir conditions. Few researches have been done with microbial enhanced oil recovery under extreme environmental conditions [18-19]. In our previous work [20], the potential of ERCPPI-2 for the production of a boisurfactant mixture with excessive oil spreading and emulsification properties under extreme environmental conditions had been studied. Besides, it was shown that ERCPPI-2 is able to recover the trapped oil in reservoir rocks.
In this study, various mechanisms of MEOR will be investigated to increase our understanding of those factors that can enhance the performance of ERCPPI-2 in the area of MEOR. These factors include: Investigation of microbial fluid effect on rock properties, Identification of good nutrient for the microbes for quick stimulation under reservoir conditions and the investigation of oil releasing mechanisms during MEOR.
2. Research Method
2.1. Chemicals
Olive oil was purchased from Vital Oil Company in Spain. Gachsaran crude oil (API=32.1) was selected from one of the main oil fields in south of Iran to represent the crude oil sample. All other chemicals were also of analytical grade and purchased from Merck Company.
2.2. Bacteria
Bacterial consortium of Enterobacter cloacae and Pseudomonas sp. which was isolated in Shiraz University Institute of Biotechnology of [20] was employed in this work
2.3. Growth conditions
Nutrient rich agar medium containing 1% yeast extract, 1.5% nutrient broth, 1%ammonium sulfate and 2% agar and Luria-Bertani (LB) medium, consisting of 1% Bacto tryptose, 0.5% Bacto yeast extract, and l% NaCl were used to culture the consortium. A minimal salt medium (MS) containing the following basal components (per liter): KH2PO4, 2.7 g, K2HPO4, 13.9 g, NaNO3, 1.0 g, NaCl, 1.0 g, yeast extract, 0.5 g was used throughout the study. The basal minimal medium was supplemented with 10 ml of a stock solution of 0.25% (w/v) MgSO4.7H2O, along with 10 ml of 1% (w/v) (NH4)2SO4 sterilized by filtration through 0.2 µm membrane filters (Millipore Corp., Bedford, MA, USA). A trace element solution (10 ml) consisting of the following composition (per 1000 ml): EDTA 0.5 g, MnSO4.H2O, 3.0g, NaCl, 1.0 g, ZnSO4.7H2O, 0.10 g. CuSO4.5H2O, 0.01 g, FeSO4.7H2O, 0.10 g, AlK(SO4)2, 0.01g, CaCl2.2H2O, 0.10 g, Na2MoO4.2H2O, 0.01 g, NiCI2.6H20, 0.003 g, H3BO3, 0.01 g was sterilized by filtration and added to the medium. Olive oil with the concentration of 0.5% (w/v) was added to the final culture. The initial pH of the medium was adjusted to 7.0.
2.4. Potential of ERCPPI-2 for biosurfactant production under in situ conditions
The success of microbial enhanced oil recovery process depends mostly on the growth of microbes and biosurfactant production under reservoir conditions. In our previous work, the potential of ERCPPI-2 for biosurfactant production under simulated reservoir conditions was studied [20]. The effects of various carbon and nitrogen sources, temperature, pressure, pH and salinity on the microorganism growth, biosurfactant production, surface activity and stability of produced biosurfactants by ERCPPI-2 were investigated and it was demonstrated that this consortium had a good ability for the reduction of IFT under extreme conditions.
2.5. Potential of ERCPPI-2 for wettability alteration under in situ conditions
The effect of ERCPPI-2 on wettability alteration of the core under in situ conditions was studied using Amott cell tests and contact angle measurements. The Amott-Harvey method was used here, as described by Anderson [21]. The method utilizes a sequence of imbibition and forced displacement tests and based on these measurements the Amott-Harvey wetting index was calculated according to the following equation:
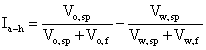 (1)
(1)
Where, Ia-h is the Amott-Harvey wetting index, Vo, sp is the volume of oil displaced by spontaneous imbibition of brine, Vo, f is the volume of oil displaced by forced imbibition of brine, Vw, sp is the volume of brine displaced by spontaneous imbibition of oil and Vw, f is the volume of brine displaced by forced imbibition of oil.
A wetting index of 1 corresponds to a strongly water-wet system, while a wetting index of –1 corresponds to a strongly oil-wet system. Wetting index equal to zero represents a neutral-wet or mixed-wet system [22].
Contact angle measurements involved a water drop coming in contact with rock surface and surrounded by kerosene. The fluids were allowed to come to equilibrium and the contact angle between the water drop and the rock surface was measured (Krüss, DSA 100, Germany). The magnitude of contact angle gives a direct indication of the rock’s wettability [21].
2.6. Fluid and rock properties
Formation brine was prepared from a solution of 1.26 g Na2SO4, 0.051 g NaHCO3, 75 g NaCl, 0.61 g KCl, 9.2 g CaCl2 and 7.6 g MgCl2 per liter. The brine was sterilized by autoclaving at 121 °C and its initial pH was adjusted to 7.0. Gachsaran crude oil under sterlie conditions was used as the oleic phase in the tests.
Dolomite outcrop cores were cut, cleaned and dried prior to the experiments. All cores had a diameter of 37.5 mm and a length of 100.0 mm. The properties of cores and different conditions that were used in oil recovery tests are listed in Table 1. Cores 1 and 2 were considered to study the microbial oil recovery (under optimal conditions) in rocks with high and low residual oil saturations, respectively. Core 3 investigated the effect of salinity with a concentration of 5% (w/v) and core 4 studied the effect of temperature at 60 °C. Core 5 had been considered for free cell-biosurfactant solution injection. In order to investigate the influence of crude oil and MS medium on wettability alteration, cores 6 and 7 were flooded with crude oil and MS medium without any bacterium injection, respectively. The results obtained from cores 6 and 7 were compared with other cores.
Table 1. Core properties and different environmental conditions in oil recovery tests.
|
Pressure (psia) |
Time duration (hours) |
Salinity (%w/v) |
Temperature (°C) |
Pore volume (cc)
|
Original Oil in Place (cc) |
Permeability (md) |
Porosity (%) |
Core |
|
2500 |
336 |
0.1 |
40 |
9.67 |
8.8 |
42.5 |
17.5 |
1 |
|
2500 |
336 |
0.1 |
40 |
7.4 |
6.4 |
37.6 |
13.4 |
2 |
|
2500 |
672 |
5.0 |
40 |
19.9 |
15.4 |
40.8 |
17.9 |
3 |
|
2500 |
672 |
0.1 |
60 |
19.1 |
14.6 |
39.2 |
17.3 |
4 |
|
2500 |
- |
0.1 |
40 |
12.24 |
10.0 |
41.7 |
22.17 |
5 |
|
2500 |
336 |
0.1 |
40 |
9.44 |
8.2 |
38.8 |
17.1 |
6 |
|
2500 |
336 |
0.1 |
40 |
9.17 |
8.1 |
37.9 |
16.6 |
7 |
2.7. Core holder flooding system
To investigate the potential of ERCPPI-2 for MEOR, the tests were performed in core holder flooding system that is shown in Figure 1. The set-up was designed so that it could simulate an oil reservoir under extreme environmental conditions. For the evaluation of oil displacement, core flooding tests were performed as described by Darvishi et al. [20].
3. Results and Analysis
3.1. Potential of ERCPPI-2 for biosurfactant production
The effect of various carbon sources (all in 0.5% w/v) on the growth, biosurfactant production and surface activity of ERCPPI-2 is shown in Figure 2. The best biosurfactant production of 1.67 g/l, interfacial tension of 0.65 mN/m and surface tension of 31.7 mN/m was obtained when the cells were grown in the presence of olive oil as the carbon source. In contact with Gachsaran crude oil, the results were 0.42 g/l, 5.75 mN/m and 41.2 mN/m, respectively.
The influence of temperature, pressure, pH and salinity on the growth, biosurfactant production, surface activity and stability of the produced biosurfactant mixture by ERCPPI-2 in contact with olive oil have been presented in Figures 3, 4, 5 and 6, respectively.
The obtained results in Figures 2-7 show that ERCPPI-2 is able to produce a biosurfactant mixture with excessive oil spreading and emulsification properties in contact with several carbon sources and a wide range of environmental conditions. Among the carbon sources that were examined, olive oil had the highest biosurfactant yield. As a result, in core flooding tests, olive oil at a concentration of 0.5% (w/v) was added to MS medium as a co-substrate of Gachsaran crude oil for the stimulation of biosurfactant production and more reduction of IFT between oil/water interfaces.
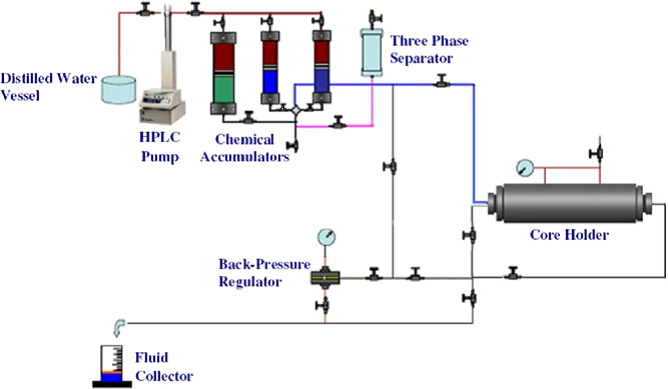
Figure 1. Core holder flooding system.
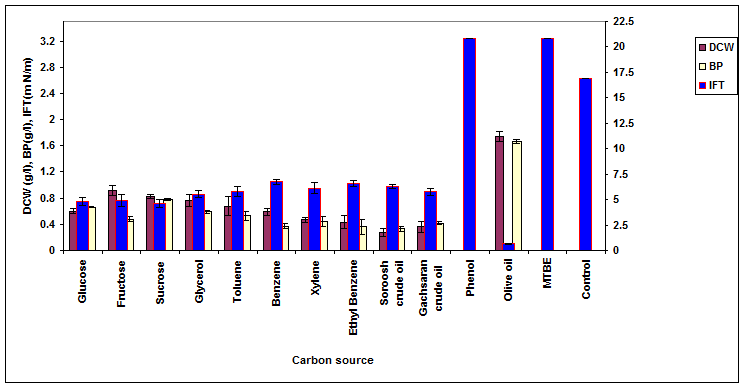
Figure 2. The effect of various carbon sources on the growth, biosurfactant production and surface activity of consortium ERCPPI-2 [20].
The reported data [20] also indicated that the produced biosurfactant mixture by ERCPPI-2 has a good stability under extreme environmental conditions and these conditions do not have any significant effect on the surface activity of the biosurfactant mixture.
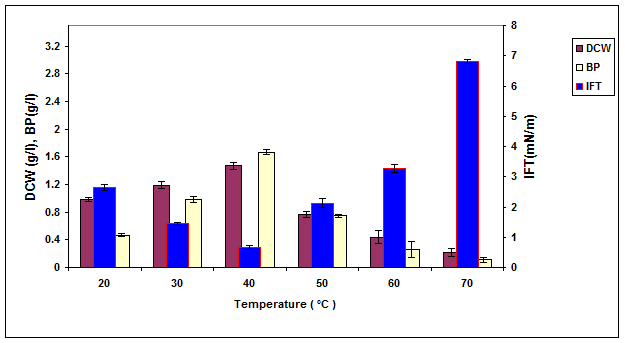
Figure 3. The effect of temperature on the growth, biosurfactant production and surface activity of consortium ERCPPI-2 [20].
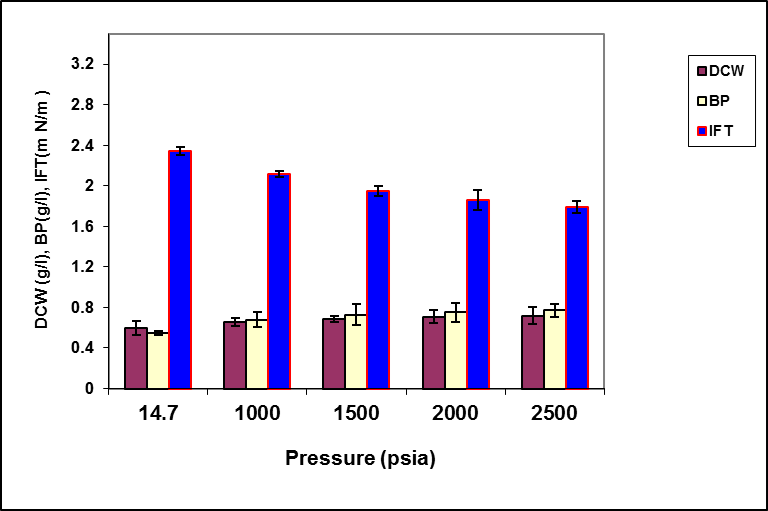
Figure 4. The effect of pressure on the growth, biosurfactant production and surface activity of consortium ERCPPI-2.
3.2. The potential of ERCPPI-2 for wettability alteration under in situ conditions
The Amott-Harvey wetting index and contact angle of core surfaces are presented in Table 2. In contact with pure outcrop, these results were 0.855 and 24.5°, respectively. It is also shown in Table 2 that cell-free biosurfactant injection had changed the wettability of core towards more oil-wet conditions.
3.3. The potential of ERCPPI-2 for enhanced oil recovery
Table 3 presents the residual oil saturation of cores before and after the microbial treatment. Besides, the percentage of oil that was recovered during the final waterflooding of cores is shown in Figures 7 to 11.
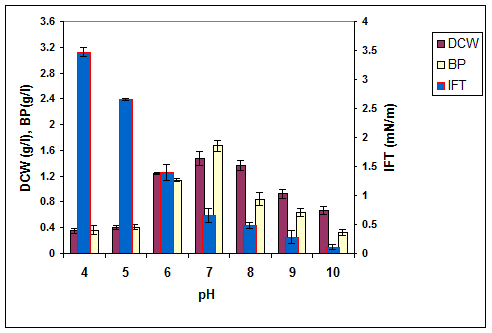
Figure 5. The effect of pH on the growth, biosurfactant production and surface activity of consortium ERCPPI-2 [20].
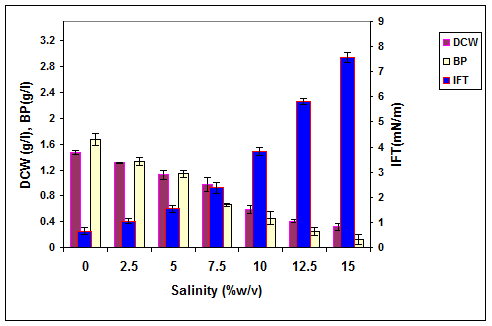
Figure 6. The effect of salinity on the growth, biosurfactant production and surface activity of consortium ERCPPI-2 [20].
Table 2. The Amott-Harvey indiex and contact angle of core surfaces.
|
Contact angle (°) |
Ia-h |
Core |
|
8.8 |
0.963 |
1 |
|
12.6 |
0.935 |
2 |
|
18.5 |
0.922 |
3 |
|
21.6 |
0.893 |
4 |
|
50.7 |
0.826 |
5 |
|
65.6 |
0.774 |
6 |
|
28.9 |
0.842 |
7 |
As shown in Figure 7, the injection of cell-free biosurfactant solution through the core at a temperature of 40 °C and pressure of 2500 psia resulted in an oil recovery of 27.2%. On the other hand, the injection of the bacterial consortium into the core at a temperature of 40 °C, pressure of 2500 psia, salinity of 0.1% (w/v) and initial oil saturations of 51.6 and 21% (two different tests at high and low residual oil saturations) resulted in an oil recovery of 48.8 and 19.1%, respectively (Figures 8 and 9). In both cases, the final residual oil saturations were reduced below 3%.
Table 3. Residual oil saturation of cores before and after the microbial injection of cores.
|
Sor after the |
Sor before the |
Core |
|
0.0454 |
0.534 |
1 |
|
0.019 |
0.21 |
2 |
|
0.423 |
0.664 |
3 |
|
0.294 |
0.685 |
4 |
|
0.302 |
0.574 |
5 |
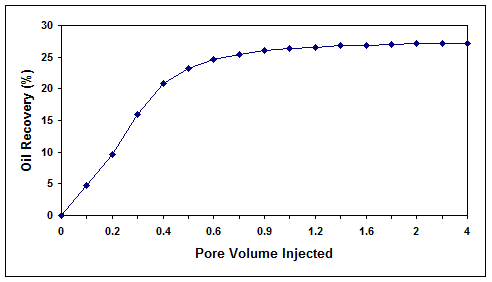
Figure 7. Oil recovery efficiency by injection of free-cell biosurfactant solution into core 5.
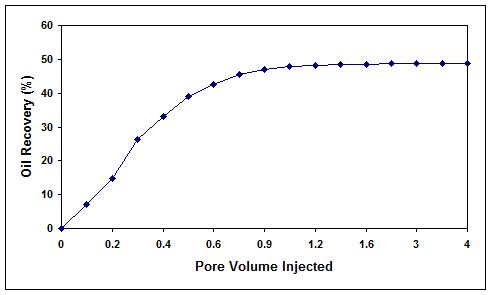
Figure 8. Microbial oil recovery efficiency for core 1 with high residual oil saturation.
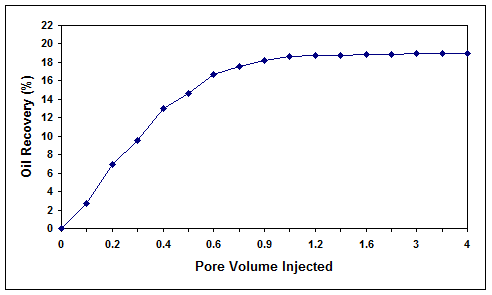
Figure 9. Microbial oil recovery efficiency for core 2 with low residual oil saturation.
In the same conditions, the test was repeated for a core with the residual oil saturation of 66.4% and salinity of 5% (w/v). As indicated in Figure 10, the oil recovery was measured to be 24.1% for this case. In the next experiment
(Fig. 11) with a residual oil saturation of 68.5%, the temperature was increased to 60 °C and salinity returned to 0.1% (w/v), which resulted in an oil recovery of 39.1%.
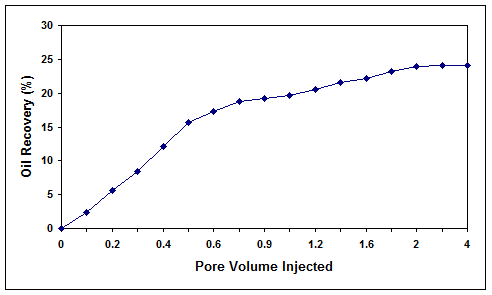
Figure 10. Microbial oil recovery efficiency for core 3 at salinity of 5% (w/v).
Under optimal conditions, ERCPPI-2 was able to reduce the residual oil saturation of cores below 3%. In more severe conditions of salinity and temperature, the oil recovery was decreased, but the adverse effect of salinity was more than the temperature. As temperature increases, viscosity reduction among the other oil recovery mechanisms plays a more effective role.
3.4. Extending the results to the Iranian reservoir field conditions
There is extensive literature on MEOR studies; however, many of these have focused on pure or isolated culture of bacteria. Data on strains that have been adapted to high salinity and temperature (even within the range of parameters of the present study) and their microbial enhanced oil recovery potentials have been rarely investigated. It is therefore important that adequate steps are taken to find the right microorganism that will proliferate under these conditions. One of the available options is the pre-adaptation of bacteria to reservoir conditions. Proper adaptation of microbes before injection will not only improve the ability of bacteria cells to withstand any physiological and environmental stress but also ensure the production of desired metabolites that can be controlled and monitored. Adaptation time is a critical factor in the overall process recovery efficiency, and should be determined in the laboratory for every proposed microbial flooding system [23-25]. The results of present work reinforced the statement that adaptation of consortium to extreme reservoir conditions of high temperature is possible to a certain extent. However, for the adaptation to be successful, it has to be through a gradual acclimation. The pure strains of ERCPPI-2 were able to tolerate higher level of temperature. Engineering of new strains of bacteria by proper adaptation to high temperatures to meet reservoir conditions in some of the Iranian oilfields is in progress. The highest adaptation temperature ever achieved in our tests was 83 °C compared to the temperature of 60 °C performed in MEOR tests using laboratory scale experiments. The obtained data clearly demonstrated the strains adapted to higher temperatures from that of pure cultures and thus improved their abilities for metabolic production at high temperature levels. It is expected that the knowledge obtained from this study can give useful information in the successful implementation of MEOR technology for the enhanced oil recovery under some of Iranian reservoir conditions.
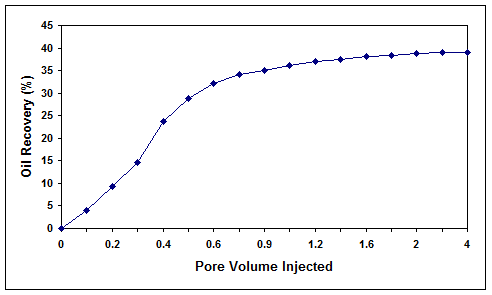
Figure 11. Microbial oil recovery efficiency for core 4 at temperature of 60 °C.
The results of present study indicated that ERCPPI-2 is a halothermotoleran consortium which is able to produce bio-products in a wide range of environmental conditions.The produced bio-products are very efficient leading to high percentage of oil recovery under reservoir conditions. Combining multiple microbial mechanisms by ERCPPI-2 has a significant effect on improving the oil recovery efficiency. Enterobacter sp. was capable of producing a high amount of gas and polysaccharide (a high molecular weight biopolymer) per mole of substrate consumed [20, 26]. Gas production has been mentioned as an important mechanism for oil recovery which increases the pressure of core sample, swells the crude oil and reduces its viscosity [27]. The role of polysaccharide biopolymer in enhanced oil recovery is to improve the volumetric sweeping efficiency of waterflood by selective plugging of high permeability zones or water-invaded zones [28]. In addition, it increases the viscosity of aqueous phase and reduces the viscosity of oil phase [20]. The simultaneous effects of increasing viscosity of the aqueous phase and decreasing viscosity of the oil phase will result in favorable mobility ratios. On the other hand, Psedomonas sp. produces rhamnolipid biosurfactcnt which has a substantial effect on the reduction of oil/water interfacial tension and recovering the trapped oil [20]. IFT reduction is responsible for the major part of oil recovery from very small pores and throats of the reservoir rocks under MEOR process [9].
The measurement of Amott-Harvey wetting index and contact angle of the cores injected by ERCPPI-2 demonstrated that bacterial growth could change the wettability of core towards more water-wet conditions which led to high oil recovery efficiencies. It was shown that wettability of minerals could be altered by the adsorption of different organic matter from crude oil and during the bacterial growth creating various types and degrees of wettability [14]. Effects of bacteria on wettability have been studied in a few publications. They reported that the change in wetting properties is dependent on the initial wetting conditions of cores. Bacterial growth in an initially oil-wet system can result in more water-wet conditions [13], while a water-wet system can change towards less water-wet conditions [14]. According to capillary pressure measurements in sand packs between glass plates, residual oil saturation decreased and a change towards more water-wet conditions was observed when bacteria were present. The mechanisms for the improvement of sweep efficiency were assumed to be the emulsification of oil, wettability alteration and gas pressurization [1, 26].
Findings of the present study indicate that properties of ERCPPI-2 in the area of MEOR are comparable to other strains introduced by similar works. Bacillus licheniformis JF-2 and Clostridium acetobutylicum were used successfully at simulated subsurface reservoir conditions, yielding an additional 17-19% oil recovery [29]. Raiders et al. [30] showed the ability of microorganisms in Berea sandstone to improve the volumetric sweep efficiency and increase oil recovery by 10-38% of the original oil in place by in situ growth and metabolism following the injection of nutrients. Yakimov et al. [18] studied the potential of several strains of Bacillus licheniformis in enhanced oil recovery and reported that this strain produces significant amount of a surfactant similar to surfactin at moderately elevated temperatures of 55 °C and salinities up to 12% NaCl. Qingxin, et al. [31] reported that Pseudomonas aeruginosa and its metabolic products could enhance the oil recovery in the model reservoir by 11.2%. In another work by Enas, et al. [32], Thermoanaerobacter brockii subsp. lactiethylicus strain 9801T originally isolated from a deep reservoir environment was evaluated for potential use in MEOR. Investigation was conducted to find the optimum environmental parameters for the growth (temperature, salinity and pH) and production of metabolites desired for microbial enhanced oil recovery. There was a growth in the media with pH from 6-9.5, salinity range of 0.5-3.5% (w/v) and temperature of 50-60 °C.
4. Conclusion
The bacterial consortium of Enterobacter cloacae and Pseudomonas sp. were investigated as a suitable candidate for the possible utilization in microbial enhanced oil recovery. The main aspects covered during the investigation were adaptation to extreme reservoir conditions, metabolites production, microbial fluid rock interactions and oil recovery mechanisms. These are based on the ability of consortium to grow in relatively harsh conditions and produce metabolites that can efficiently mobilize the trapped oil. It is evident that this consortium offer useful metabolic products such as biosurfactant, biopolymer, biogas, in addition to biomass for enhancing the oil recovery. The produced bio-products can contribute to multiple microbial mechanisms which can tackle specific problems of oil recovery. Through this, it will be possible to effectively control, monitor and utilize microbial activities in the recovery and utilization of oil resources. The findings also indicate that the range of temperature at which this consortium could grow is low compared to conditions of many Iranian oil wells. However, the results are comparable to other microbial injection tests that have been presented in other reports and they can provide an opportunity for further research in terms of its applications in microbial enhanced oil recovery. Engineering the new strains of ERCPPI-2 by proper adaptation to meet reservoir conditions in the Iranian oilfields is in progress.